European Union
In the European Union, the inspection of both sub-visible and visible particles in parenteral products is governed by the European Medicines Agency (EMA) and the European Pharmacopoeia, with detailed guidelines ensuring the safety and quality of injectable medications. Here’s a comprehensive overview of the regulations:
Visible Particles
- European Pharmacopoeia: The European Pharmacopoeia requires that parenteral products be “essentially free from visible particles.” This is interpreted as no unit should contain any visible particles when inspected under suitable conditions.
- Manual and Automated Inspection: Both manual and automated systems are used for the inspection of visible particles. The use of automated systems is increasing, but these systems must be validated to ensure they are as effective as manual inspection (GMP Journal) (ECA Academy) (GMP Journal).
- GMP Guidelines: The EU’s Good Manufacturing Practice (GMP) guidelines require regular qualification and requalification of inspection systems and operators to ensure ongoing compliance. This includes functional testing and periodic evaluation to confirm the systems’ performance.
- the Ph. Eur. includes monographs and standards for visible particles in parenteral preparations. Chapter 2.9.20 covers particulate contamination and provides methods for inspection.
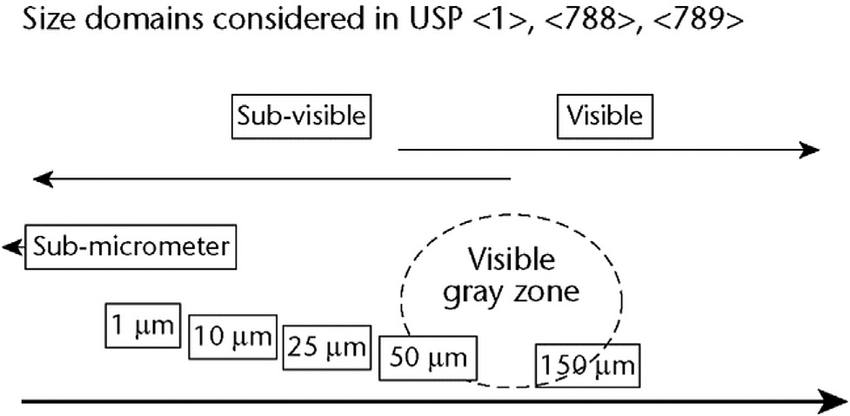
Sub-visible Particles
- European Pharmacopoeia Monograph 2.9.19: This monograph specifies the methods for testing sub-visible particulate contamination in injectable products. The limits are:
- For large volume parenterals (LVPs): Not more than 25 particles per milliliter equal to or greater than 10 micrometers, and not more than 3 particles per milliliter equal to or greater than 25 micrometers.
- For small volume parenterals (SVPs): Not more than 6,000 particles per container equal to or greater than 10 micrometers, and not more than 600 particles per container equal to or greater than 25 micrometers (GMP Journal).
- Testing Methods: The primary methods for detecting sub-visible particles include Light Obscuration Particle Count Test and Microscopic Particle Count Test. These methods are designed to detect and quantify particles in the size range that could potentially pose a risk to patients.
Japan
In Japan, the regulation of sub-visible and visible particles in parenteral products is overseen by the Pharmaceuticals and Medical Devices Agency (PMDA), aligning closely with international standards such as those of the US FDA and European EMA. The Japanese Pharmacopoeia (JP) sets specific requirements for the inspection and control of particulate matter in injectable products. Here’s an overview of the regulations:
Visible Particles
- Japanese Pharmacopoeia: The JP mandates that parenteral products be free from visible particles. This requirement is akin to the standards set by the United States Pharmacopeia (USP) and European Pharmacopoeia.
- Visual Inspection: All parenteral products must undergo 100% visual inspection. This can be done manually or with automated systems, both of which must be validated to ensure they can reliably detect visible particles. The inspection conditions, including lighting and background, must be standardized to ensure consistency and accuracy (ECA Academy).
Sub-visible Particles
- Japanese Pharmacopoeia: The JP specifies the acceptable limits and methods for testing sub-visible particulate matter in parenteral products.
- Testing Methods: The primary methods include Light Obscuration Particle Count Test and Microscopic Particle Count Test, similar to those specified in the USP.
- Limits for Sub-visible Particles:
- For large volume parenterals (LVPs): Not more than 25 particles per milliliter equal to or greater than 10 micrometers, and not more than 3 particles per milliliter equal to or greater than 25 micrometers.
- For small volume parenterals (SVPs): Not more than 6,000 particles per container equal to or greater than 10 micrometers, and not more than 600 particles per container equal to or greater than 25 micrometers (ECA Academy).
Regulatory Oversight
- PMDA Guidelines: The PMDA ensures compliance with these standards through inspections and regulatory oversight. Manufacturers must adhere to Good Manufacturing Practice (GMP) standards, which include rigorous testing for particulate matter in parenteral products.
- ICH Harmonization: Japan, as part of the International Council for Harmonisation (ICH), adopts harmonized guidelines like ICH Q4B Annex 3, which standardizes the tests for particulate contamination across ICH regions. This helps ensure that methods and limits for sub-visible particles are consistent internationally (European Medicines Agency).
Risk Management
- Risk-Based Approach: The Japanese regulatory framework emphasizes a risk-based approach to managing particulate contamination. This involves identifying potential sources of particulate matter throughout the manufacturing process and implementing controls to mitigate these risks. Continuous monitoring and periodic re-evaluation of inspection processes are essential components of this approach (PharmOut) (Default).
Key Points
- 100% Visual Inspection: Every unit of parenteral product must be visually inspected to ensure it is free from visible particles. This inspection must be conducted under standardized conditions.
- Qualification and Requalification: Both manual and automated inspection systems must be qualified and periodically requalified to ensure consistent performance in detecting visible and sub-visible particles.
- Sub-visible Particle Testing: Regular testing for sub-visible particles must be performed using standardized methods, with specified limits for particle sizes.
- Documentation and Compliance: Manufacturers are required to maintain detailed records of inspection results and ensure compliance with JP standards and PMDA guidelines to guarantee the safety and quality of injectable products.
By following these regulations, Japan ensures that parenteral products meet stringent safety and quality standards, protecting patient health and ensuring the efficacy of injectable medication
ICH
The International Council for Harmonisation (ICH) provides comprehensive guidelines for the inspection of sub-visible and visible particles in parenteral products, ensuring consistency and high standards across different regulatory regions. The relevant ICH guidelines are primarily found in the Q4B series and other quality-related guidelines.
Key ICH Guidelines
- ICH Q4B Annex 3: Evaluation and Recommendation of Pharmacopoeial Texts for Use in the ICH Regions on Test for Particulate Contamination: Sub-visible Particles:
- This guideline harmonizes the methods for testing sub-visible particles in injectable products among ICH regions (EU, Japan, and the US).
- It facilitates the mutual recognition of pharmacopoeial procedures, ensuring that methods described in the United States Pharmacopeia (USP), European Pharmacopoeia (Ph. Eur.), and Japanese Pharmacopoeia (JP) are interchangeable without re-validation in different regions (European Medicines Agency).
- ICH Q8 (R2) Pharmaceutical Development:
- Although not specifically focused on particulate matter, ICH Q8 (R2) discusses the principles of pharmaceutical development, including the design of robust manufacturing processes that can consistently produce products meeting predefined quality criteria, including particulate contamination limits (PharmOut).
- ICH Q9 Quality Risk Management:
- This guideline emphasizes a risk-based approach to managing quality, including the risk of particulate contamination in parenteral products. It provides a framework for identifying, assessing, and controlling risks throughout the product lifecycle (Default).
- ICH Q10 Pharmaceutical Quality System:
- ICH Q10 provides a comprehensive model for a pharmaceutical quality system, which includes the management of processes that affect product quality, such as the control of particulate matter in parenteral products. It integrates principles from ICH Q8 and Q9 to ensure continuous improvement and regulatory compliance (Default).
Harmonized Testing Methods
- Light Obscuration Particle Count Test:
- This method is commonly used for routine testing of sub-visible particles. It detects and counts particles based on the amount of light blocked by the particles in a sample.
- Microscopic Particle Count Test:
- This method is used when samples cannot be analyzed by light obscuration, such as those containing proteins or other substances that may interfere with light measurement. It involves counting particles under a microscope.
Limits for Sub-visible Particles
- Large Volume Parenterals (LVPs):
- Not more than 25 particles per milliliter equal to or greater than 10 micrometers, and not more than 3 particles per milliliter equal to or greater than 25 micrometers.
- Small Volume Parenterals (SVPs):
- Not more than 6,000 particles per container equal to or greater than 10 micrometers, and not more than 600 particles per container equal to or greater than 25 micrometers (European Medicines Agency).
Visible Particle Inspection
- 100% Visual Inspection: All parenteral products must undergo 100% visual inspection for visible particles. Both manual and automated inspection methods are accepted, provided they are validated to be effective.
Risk Management and Quality Assurance
- Risk-Based Approach: ICH Q9 emphasizes the need for a risk-based approach to identify and control potential sources of particulate contamination.
- Quality Management Systems: ICH Q10 outlines the importance of a robust quality management system to ensure continuous monitoring, control, and improvement of manufacturing processes related to particulate contamination.
Regulatory Consistency
By adopting these ICH guidelines, regulatory authorities in the EU, Japan, and the US ensure that their standards for particulate contamination in parenteral products are aligned, facilitating smoother international trade and regulatory processes. This harmonization helps maintain high standards of safety and efficacy for injectable products globally.
These guidelines collectively ensure that parenteral products meet stringent safety and quality standards, minimizing the risk of particulate contamination and protecting patient health across ICH regions.
Inspection Machine