Regulation and Guideline of particles inspection in parenteral products in USA
In the United States, the regulation of sub-visible and visible particle inspection in parenteral products is primarily governed by the Food and Drug Administration (FDA) and outlined in the United States Pharmacopeia (USP). These regulations ensure the safety and quality of injectable medications through stringent testing and inspection protocols. Here’s an overview of the key regulations:
Visible Particles
- FDA Requirements: The FDA mandates that parenteral products be “essentially free” of visible particles. This requirement is enforced through various guidelines and the United States Pharmacopeia (USP).
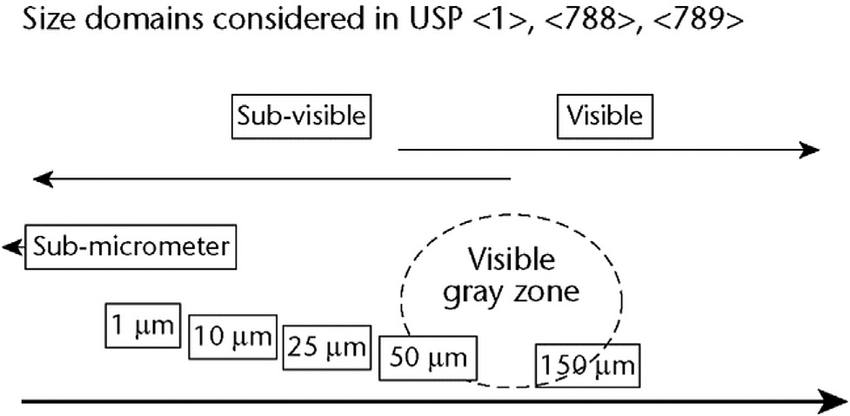
- USP <1>: General chapter <1> outlines the basic requirements for injectable products, including the need to be free from visible particulates (ECA Academy).
- USP <790>: This chapter provides detailed guidelines for the visual inspection of parenteral products. It mandates 100% visual inspection of all units to detect and remove those containing visible particles. The chapter also describes the conditions under which inspections should be conducted to ensure accuracy and consistency (FDA) (ECA Academy).
- USP <1790>: This chapter provides guidance on the qualification and validation of inspection processes, including both manual and automated methods (FDA) (ECA Academy).
- FDA Guidance: The FDA has also issued a draft guidance document titled “Inspection of Injectable Products for Visible Particulates,” emphasizing the importance of a robust inspection process and the use of risk assessment and control strategies to minimize particulate contamination (FDA).
Sub-visible Particles
- USP <788>: This chapter of the USP outlines the test methods and limits for sub-visible particulate matter in injections and parenteral infusions. It specifies two primary test methods:
- Light Obscuration Particle Count Test: Used for routine analysis, it measures particles in the range of 2 micrometers to 100 micrometers.
- Microscopic Particle Count Test: Used for samples that cannot be analyzed by light obscuration, it involves counting particles under a microscope.
- Limits for Sub-visible Particles:
- For large volume parenterals (LVPs): Not more than 25 particles per milliliter equal to or greater than 10 micrometers, and not more than 3 particles per milliliter equal to or greater than 25 micrometers.
- For small volume parenterals (SVPs): Not more than 6,000 particles per container equal to or greater than 10 micrometers, and not more than 600 particles per container equal to or greater than 25 micrometers (FDA) (ECA Academy).
Regulatory Oversight
- FDA Oversight: The FDA ensures compliance with these standards through regular inspections and enforcement actions. Manufacturers must adhere to Current Good Manufacturing Practice (CGMP) regulations, which include rigorous testing for particulate matter in parenteral products.
- Risk Management: The FDA emphasizes a risk-based approach to particulate contamination, involving the identification, assessment, and control of potential sources of particles throughout the manufacturing process. This includes implementing robust quality control measures and regular monitoring to minimize the risk of contamination (FDA) (ECA Academy).
Key Points
- 100% Visual Inspection: All parenteral products must undergo 100% visual inspection for visible particles, with inspections conducted under suitable lighting and environmental conditions to ensure accuracy.
- Qualification and Requalification: Inspectors and inspection systems must be regularly qualified and requalified to maintain high standards of detection reliability. This includes both manual and automated inspection systems.
- Sub-visible Particle Testing: Routine testing for sub-visible particles must be conducted using light obscuration and microscopic methods, with specified limits for particle sizes in both large and small volume parenterals.
- Documentation and Compliance: Manufacturers are required to document inspection results and maintain compliance with USP standards and FDA guidelines to ensure the safety and quality of injectable products.
Visual Inspection Machine For IV PP bottle