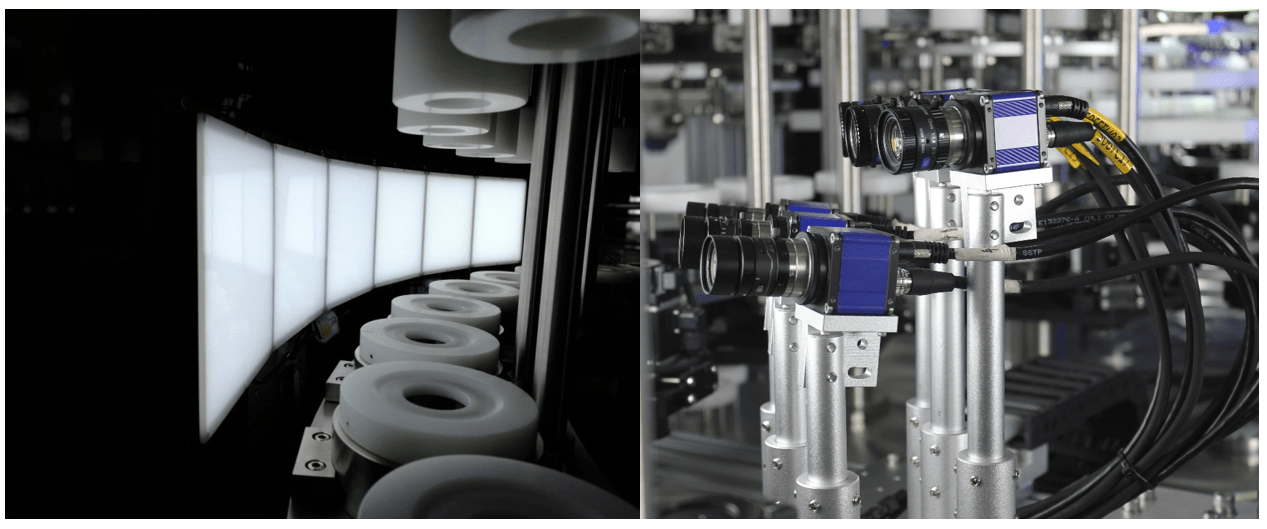
IV bag inspection machines play a critical role in the pharmaceutical industry by ensuring that IV bags are free from defects and meet quality standards before they are used for patient treatment. These machines are vital for machine validation, automated inspection, and quality control in pharmaceutical inspection processes.
Automated inspection systems are essential for conducting safety checks and sterile processing within the pharmaceutical industry. IV bag inspection machines are an integral part of sterile product inspection, ensuring that solutions, medications, and infusion bags are free from contamination. These machines help in detecting defects, ensuring the accuracy of inspection, and maintaining high speed inspection on the production line.
With the use of visual inspection machines, pharmaceutical companies can guarantee the quality of IV bag manufacturing and packaging. These machines aid in process control, detecting particles, and ensuring seal integrity in IV bags. IV bag inspection equipment plays a crucial role in maintaining pharmaceutical industry standards and upholding IV bag production practices.
Automation in pharmaceuticals is made possible by IV bag inspection machines, which enable pharmaceutical companies to conduct thorough inspections efficiently. By utilizing IV bag inspection machines, pharmaceutical companies can enhance their quality assurance processes, minimize errors, and improve overall product safety in the pharmaceutical industry.
Understanding the Importance of IV Bag Inspection
IV Bag Inspection is a critical process in the pharmaceutical industry, ensuring the safety and quality of intravenous solutions. Automated inspection machines play a vital role in conducting thorough safety checks, sterile processing, and quality control of IV bags during the manufacturing and production line inspection.
These visual inspection machines are designed to detect defects, contamination, and ensure seal integrity with high speed and accuracy. By incorporating automation in pharmaceuticals, IV bag inspection guarantees that sterile products meet pharmaceutical industry standards and comply with strict regulations.
The importance of IV bag inspection lies in guaranteeing the quality assurance of pharmaceutical packaging, specifically in solution and infusion bags. By conducting particle inspection and contamination detection, these machines ensure that every IV bag produced is free from any impurities that could compromise the patient’s safety.
In conclusion, IV bag inspection is a crucial step in the production process, providing a reliable method for ensuring the quality and safety of IV bags in the pharmaceutical industry.
Automation in Pharmaceuticals: The Rise of IV Bag Inspection Machines
Automation in Pharmaceuticals has revolutionized the industry, particularly in the realm of IV Bag Inspection Machines. These machines play a pivotal role in ensuring the quality, safety, and accuracy of IV bags used in healthcare settings. Machine validation is a critical aspect of automated inspection, as it guarantees that the equipment meets pharmaceutical industry standards.
Pharmaceutical inspection involves a series of safety checks and sterile processing to maintain the integrity of the products. IV bag manufacturing requires meticulous production line inspection to detect any defects that may compromise the quality of the final product. Visual inspection machines are designed for high speed inspection with unparalleled accuracy in detecting contamination or other issues.
One of the key functions of IV bag inspection machines is to check for seal integrity and particle inspection to prevent any contamination in the sterile product. These machines are equipped with advanced technology to facilitate solution bag inspection and infusion bag inspection, ensuring IV bag quality assurance at every stage of production.
Automation in pharmaceuticals has significantly improved process control and efficiency in the pharmaceutical industry. IV bag inspection machines have streamlined the inspection process, reducing human error and increasing the reliability of inspection results. With automation, pharmaceutical companies can meet the demands for high quality products while adhering to stringent regulations.
In conclusion, the rise of IV Bag Inspection Machines signifies a new era in pharmaceutical inspection, where automation plays a crucial role in maintaining quality and safety standards. Embracing this technological advancement is essential for pharmaceutical companies looking to enhance their production processes and ensure the delivery of safe and reliable products to consumers.
Ensuring Quality Control and Safety Checks with Advanced Inspection Equipment
Automated inspection machines play a crucial role in ensuring the quality control and safety of IV bags in the pharmaceutical industry. These high speed visual inspection machines are designed to detect defects, contamination, and seal integrity issues in IV bag manufacturing. By utilizing advanced inspection equipment, pharmaceutical companies can maintain sterile processing, adhere to industry standards, and guarantee the quality and safety of their products.
Machine validation is an essential step in the pharmaceutical inspection process, as it verifies the accuracy and reliability of the inspection equipment. Through automation in pharmaceuticals, IV bag production lines can achieve efficient and precise inspection processes, minimizing the risk of errors and contamination. These inspection machines are equipped with solutions for particle inspection, solution bag inspection, and infusion bag inspection, ensuring thorough quality assurance in the production line.
Quality control and safety checks are paramount in the pharmaceutical industry, and inspection equipment plays a critical role in upholding these standards. By utilizing visual inspection machines, pharmaceutical companies can detect and eliminate any defects or contaminants in IV bags, ensuring the sterile product inspection and compliance with pharmaceutical packaging requirements. The accuracy in inspection provided by these machines helps maintain the integrity of IV bag production and guarantees the safety of patients receiving intravenous medications.
In conclusion, advanced inspection equipment is essential for ensuring quality control and safety checks in IV bag manufacturing. By incorporating automated inspection machines into production lines, pharmaceutical companies can detect defects, ensure sterile processing, and maintain compliance with industry standards. With the use of high speed inspection and precise defect detection, these machines play a vital role in upholding the quality and safety of pharmaceutical products.
Key Features and Capabilities of Automated IV Bag Inspection Machines
Automated IV bag inspection machines offer a wide range of key features and capabilities that are crucial for ensuring pharmaceutical inspection, quality control, and safety checks in the production of IV bags. These machines are designed to perform automated inspections of IV bag manufacturing processes, including defect detection, process control, and contamination detection. Here are some of the main features and capabilities of these high tech machines:
- Machine validation: Automated IV bag inspection machines are equipped with advanced technology that ensures accurate and reliable inspection results, in compliance with pharmaceutical industry standards.
- Sterile processing: These machines are designed to maintain sterile conditions during the inspection process, ensuring the safety and quality of IV bag production.
- High speed inspection: Automated IV bag inspection machines can conduct inspections at high speeds, allowing for efficient and rapid detection of any defects or contaminants.
- Accuracy in inspection: These machines are capable of detecting even the smallest particles or imperfections in IV bags, ensuring high levels of accuracy in inspection results.
- Seal integrity: Automated IV bag inspection machines are equipped with tools to check the seal integrity of IV bags, ensuring that the contents remain sterile and secure.
- IV bag quality assurance: These machines play a crucial role in quality assurance processes, helping to identify and eliminate any issues in IV bag production.
- Automation in pharmaceuticals: Automated IV bag inspection machines streamline the inspection process, reducing the need for manual intervention and increasing efficiency in pharmaceutical manufacturing.
- Infusion bag inspection: These machines are capable of inspecting various types of IV bags, including solution bags and infusion bags, to ensure the quality and safety of pharmaceutical packaging.
- Pharmaceutical machinery: Automated IV bag inspection machines are an essential component of pharmaceutical machinery, playing a critical role in ensuring the integrity and safety of sterile products.
In conclusion, automated IV bag inspection machines offer a wide range of sophisticated features and capabilities that are essential for maintaining the highest standards of quality and safety in pharmaceutical production. With their advanced technology and precision, these machines play a crucial role in the pharmaceutical industry, contributing to the delivery of safe and effective IV bag products to patients worldwide.
The Process of Machine Validation in Pharmaceuticals
The process of machine validation in pharmaceuticals involves ensuring the accuracy and reliability of IV bag inspection machines. These automated inspection systems play a crucial role in quality control and safety checks in sterile processing within the pharmaceutical industry.
- Pharmaceutical inspection requires strict adherence to industry standards to guarantee the safety and efficacy of pharmaceutical products.
- IV bag inspection machines are essential for detecting defects in IV bag manufacturing and ensuring sterile product inspection along the production line.
- Visual inspection machines are used for high speed inspection to detect contamination, particle inspection, seal integrity, and solution bag inspection.
- Automation in pharmaceuticals has revolutionized the inspection process, enhancing accuracy in inspection and speeding up production lines.
- Inspection equipment employs advanced technology to ensure IV bag quality assurance and compliance with pharmaceutical industry standards.
Machine validation in pharmaceuticals is a critical process to maintain the integrity of IV bag production and pharmaceutical packaging. By incorporating automated inspection systems, pharmaceutical companies can effectively detect defects, ensure sterile processing, and uphold quality control measures in the production of IV bags. This ensures that pharmaceutical products meet stringent safety and quality standards, providing patients with safe and effective medications.
Challenges and Solutions in IV Bag Manufacturing and Inspection
IV bag manufacturing and inspection present unique challenges in the pharmaceutical industry. Machine validation is crucial to ensuring automated inspection processes are accurate and reliable. Quality control measures must be in place to conduct safety checks and sterile processing. Inspection machines play a vital role in pharmaceutical manufacturing, detecting defects and contamination to maintain high standards of production.
Visual inspection machines are used for process control, conducting high speed inspections with precision. They detect particles and ensure seal integrity, crucial for sterile product inspection. Inspection equipment is essential for pharmaceutical packaging, guaranteeing the quality of IV bags before they reach consumers.
In the pharmaceutical industry, adherence to standards is paramount. IV bag production requires automation to streamline processes and enhance efficiency. Solutions in IV bag inspection involve implementing advanced technology to detect any deviations from quality standards promptly.
Overall, ensuring the accuracy of inspection processes is vital for maintaining the quality and safety of pharmaceutical products. IV bag inspection plays a significant role in the overall quality assurance of the pharmaceutical industry, making it a critical aspect of production.
Vision Inspection Machine for IV bag