In the fast-paced world of medical disposable and pharmaceutical manufacturing, ensuring the highest quality standards is paramount. One crucial aspect of maintaining these standards is through rigorous inspection techniques. In this comprehensive guide, we’ll delve into the various aspects of inspection processes, automated inspection systems, defect identification, and the importance of adherence to inspection criteria and standards.
Understanding Inspection Techniques
Inspection techniques form the backbone of quality control in manufacturing. It involves a systematic examination of products to ensure they meet predefined criteria. In the medical disposable and pharmaceutical sectors, where precision and accuracy are non-negotiable, inspection techniques play an even more critical role.
Automated Inspection: The Future of Quality Assurance
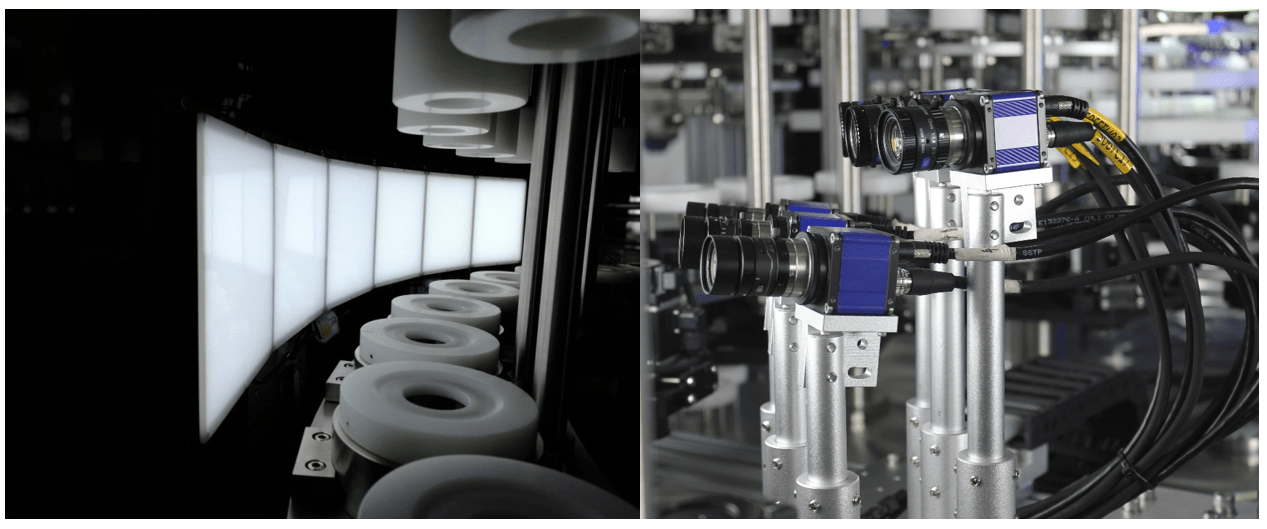
Manual inspection processes are not only time-consuming but also prone to errors. Automated inspection systems revolutionize quality assurance by leveraging advanced technologies such as machine vision and artificial intelligence. These systems offer unparalleled speed, accuracy, and consistency, making them indispensable in modern manufacturing facilities.
The Inspection Process Demystified
The inspection process comprises several stages, from initial setup to final evaluation. Each stage is meticulously designed to detect and eliminate defects effectively. By automating repetitive tasks and employing sophisticated algorithms, manufacturers can streamline the inspection process while maintaining the highest standards of quality.
Defect Identification: Spotting Imperfections with Precision
Identifying defects in medical disposable and pharmaceutical products requires a keen eye and attention to detail. Whether it’s detecting microscopic irregularities or subtle variations in color and texture, advanced inspection techniques empower manufacturers to identify defects swiftly and accurately, minimizing the risk of faulty products reaching the market.
Setting Inspection Criteria and Standards
Establishing clear inspection criteria and standards is essential for maintaining consistency and uniformity across production batches. These criteria serve as benchmarks against which products are evaluated, ensuring compliance with regulatory requirements and customer expectations. By aligning inspection criteria with industry standards, manufacturers demonstrate their commitment to quality and safety.
The Role of Product Inspection in Ensuring Compliance
Product inspection is not just about identifying defects; it’s also about ensuring compliance with regulatory guidelines and industry standards. From cleanliness and sterility to dimensional accuracy and packaging integrity, every aspect of the product undergoes rigorous scrutiny. By implementing robust inspection protocols, manufacturers can mitigate risks and safeguard their reputation.
Achieving Precision: The Importance of Inspection Accuracy
In manufacturing, precision is paramount. Even the slightest deviation from specifications can have serious consequences, especially in the medical and pharmaceutical sectors. Automated inspection machines equipped with cutting-edge technologies deliver unparalleled accuracy, enabling manufacturers to detect even the most minute defects with confidence.
Vision Inspection Machines: Enhancing Quality Control Efforts
Vision inspection machines represent the pinnacle of inspection technology. By capturing high-resolution images and analyzing them in real-time, these machines can identify defects with unmatched speed and precision. Whether it’s inspecting labels, seals, or product components, vision inspection machines offer unparalleled reliability in quality control.
Embracing Automation for Enhanced Efficiency
In today’s competitive landscape, manufacturers must leverage automation to stay ahead. Automated inspection machines not only enhance quality control efforts but also improve overall efficiency and productivity. By minimizing manual intervention and optimizing workflows, manufacturers can reduce costs, accelerate time-to-market, and ensure customer satisfaction.
Conclusion
In the dynamic world of medical disposable and pharmaceutical manufacturing, maintaining the highest quality standards is non-negotiable. By embracing advanced inspection techniques, leveraging automated inspection systems, and adhering to strict inspection criteria and standards, manufacturers can uphold their commitment to excellence while safeguarding public health and safety. In an industry where precision and accuracy are paramount, investing in robust inspection processes is not just a choice but a necessity for long-term success.