Introduction
Aerosol valves play a critical role in pharmaceutical packaging, ensuring the safe and precise delivery of medications to patients. Understanding the technical specifications of these valves is essential for maintaining product integrity and efficacy. Additionally, selecting appropriate packaging materials and assembly machines is vital in the pharmaceutical industry to meet regulatory standards and ensure product quality.
Critical Components of Pharmaceutical Aerosol Valves
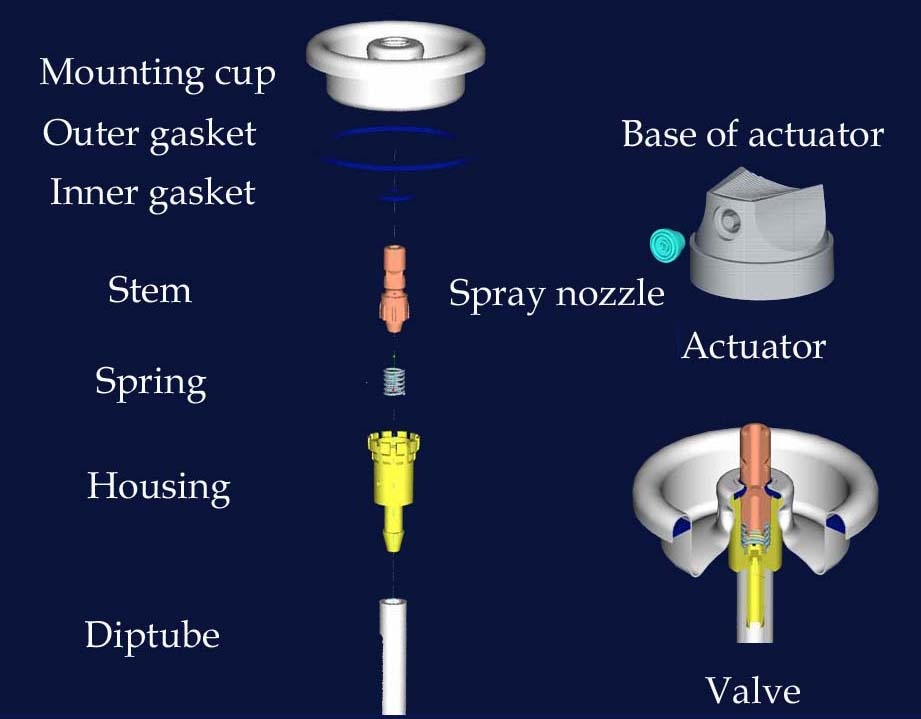
Valve Stem: The valve stem is at the heart of the aerosol valve assembly. This component controls the medication’s release by regulating the fuel flow. Its design and material are carefully selected to ensure smooth operation and compatibility with various formulations.
Internal Gasket: The internal gasket forms a tight seal within the valve, preventing leakage and maintaining the integrity of the product. It is typically made of elastomeric materials that resist corrosion and can withstand the pressure exerted during dispensing.
External Gasket: The external gasket complements the internal gasket and provides additional protection against leaks. Positioned outside the valve, it helps seal the connection between the valve and the container, ensuring the medication remains intact until use.
Mounting Housing: The mounting housing is the interface between the valve assembly and the container. It provides structural support and facilitates the secure attachment of the valve to the aerosol can or bottle. The mounting housing design is critical for ensuring proper alignment and functionality.
Spring: The spring plays a pivotal role in the operation of the aerosol valve assembly. It exerts pressure on the valve stem, ensuring proper closure when not in use and facilitating smooth dispensing when activated. Selecting the spring material and tension is crucial to achieving consistent performance.
Housing: The housing encases the internal components of the aerosol valve assembly, protecting external factors such as moisture and contaminants. It is designed to withstand the rigors of handling and transportation while maintaining the medication’s integrity.
Dip Tube: In aerosol delivery systems, the dip tube extends from the valve assembly into the product container, allowing the medication to be dispensed efficiently. It ensures that the fuel reaches the bottom of the container, enabling consistent dispensing throughout the product’s lifespan.
Actuator: The actuator is the interface between the user and the aerosol valve assembly, facilitating medication dispensing. Its design may vary depending on the application, from simple press buttons to more complex nozzle designs for targeted delivery.

Each component plays a crucial role in the functionality and reliability of aerosol valves, ensuring accurate dosing and consistent performance.
Materials Used in Pharmaceutical Aerosol Valves
1. Common materials: Aluminum, stainless steel, and plastics are frequently used for their durability and compatibility with pharmaceutical formulations.
2. Properties and considerations: Material selection is based on chemical resistance, compatibility with propellants, and ease of manufacturing.
3. Impact on product safety: The choice of materials directly influences the safety and efficacy of pharmaceutical products, highlighting the importance of thorough material selection processes.
Design Considerations for Pharmaceutical Aerosol Valves
1. Valve size and shape: Tailoring the design to suit the specific application ensures optimal performance and user experience.
2.*Flow rate and dosage accuracy: Precise control over dosing is essential for medications requiring accurate administration.
3. Actuation force and ergonomics: User-friendly design enhances usability and patient compliance.
4. Sealing mechanisms: Effective sealing prevents leakage and maintains product integrity.
5. Optimizing performance: Design modifications can improve product performance and reliability.
Applications of Pharmaceutical Aerosol Valves
1. Metered-dose inhaler
2. Nasal sprays
3. Topical aerosols
4. Oral sprays
5. Tailored specifications: Each application requires specific technical specifications to meet the needs of patients and healthcare professionals.
Assembly Machines for Pharmaceutical Aerosol Valves
1. Precision and automation: Automated assembly machines ensure consistency and efficiency in valve production.
2. Compliance with specifications: Machines are calibrated to meet technical specifications and regulatory requirements, guaranteeing product quality and safety.
3.*Role in production: Assembly machines streamline manufacturing, reducing errors and increasing output.
Contribution to Meeting Technical Specifications
1. Integration of materials and machines: integration ensures the production of high-quality aerosol valves that meet technical specifications.
2. Quality control: Robust measures are implemented throughout production to maintain consistency and compliance.
3. Impact on product performance: Packaging materials and assembly machines directly influence the performance and reliability of pharmaceutical aerosol valves, ultimately enhancing patient outcomes.
Conclusion
Understanding pharmaceutical aerosol valves’ components, materials, and design considerations is essential for ensuring product quality and patient safety. By prioritizing technical specifications and leveraging appropriate packaging materials and assembly machines, pharmaceutical companies can deliver reliable and effective medications worldwide.


