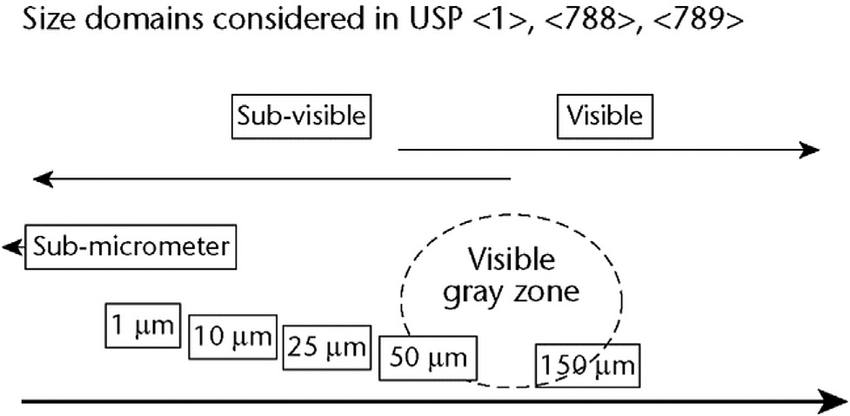
The regulation and Guideline of particles inspection in EU ,Japan ,ICH
European Union In the European Union, the inspection of both sub-visible and visible particles in parenteral products is governed by the European Medicines Agency (EMA) and the European Pharmacopoeia, with detailed guidelines ensuring the safety and quality of injectable medications. Here’s a comprehensive overview of the regulations: Visible Particles Sub-visible Particles Japan In Japan, the […]