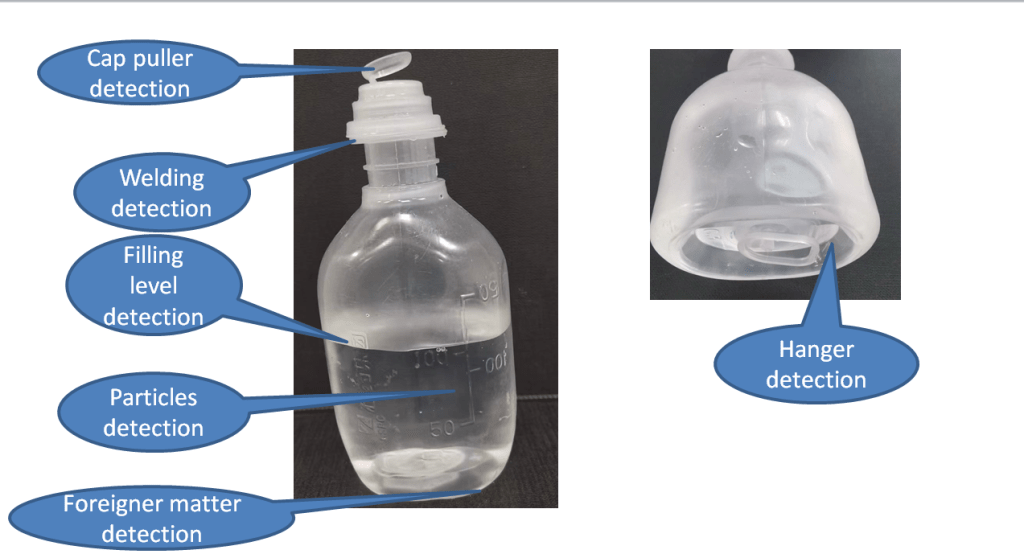
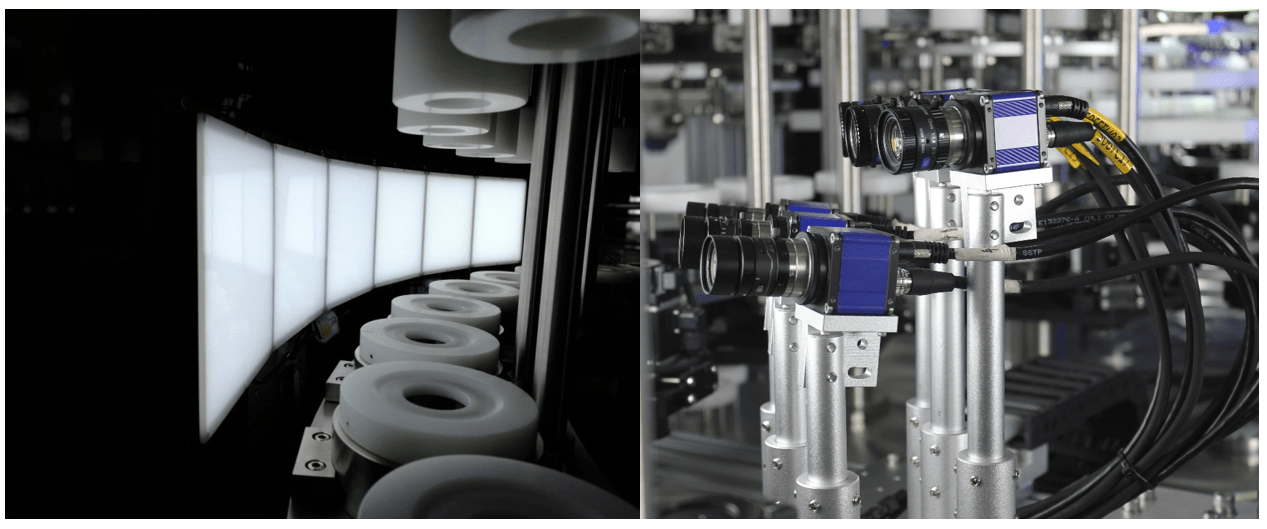
In the dynamic world of pharmaceutical manufacturing, ensuring the integrity of every bottle is paramount to maintaining product safety and compliance. Automated bottle inspection systems, powered by machine vision technology, are revolutionizing the quality control process, offering unmatched accuracy, efficiency, and reliability. In this article, we explore the significance of automated bottle inspection and its role in upholding industry standards.
Automated Bottle Inspection: Redefining Quality Assurance
Automated bottle inspection represents a quantum leap in pharmaceutical quality control, leveraging cutting-edge machine vision technology to scrutinize bottles with unparalleled precision and efficiency. By automating the inspection process, these systems eliminate the risk of human error and ensure consistent results, thereby enhancing product integrity and regulatory compliance. From detecting defects to verifying label accuracy, automated bottle inspection systems play a crucial role in safeguarding the quality and safety of pharmaceutical products.
Machine Vision Bottle Inspection: The Future of Quality Control
Machine vision technology lies at the heart of automated bottle inspection systems, enabling them to analyze visual data with remarkable speed and accuracy. By capturing high-resolution images of each bottle and processing them in real-time, machine vision algorithms can identify defects, measure dimensions, and detect inconsistencies with unmatched precision. Whether it’s inspecting glass vials for cracks or plastic containers for contaminants, machine vision bottle inspection systems set new standards for quality control in pharmaceutical manufacturing.
The Bottle Inspection Process: From Start to Finish
The bottle inspection process encompasses a series of steps designed to ensure that every container meets the highest standards of quality and safety. From initial setup and calibration to final inspection and validation, each stage plays a crucial role in maintaining product integrity and regulatory compliance. Automated bottle inspection systems streamline this process by automating repetitive tasks, minimizing human intervention, and providing real-time feedback on inspection results. By optimizing the efficiency and accuracy of the inspection process, these systems enable pharmaceutical manufacturers to achieve greater productivity and consistency in their operations.
Innovative Techniques in Bottle Inspection
Bottle inspection techniques continue to evolve as technology advances and industry standards evolve. Today, pharmaceutical manufacturers have access to a wide range of innovative techniques for inspecting bottles, including optical imaging, laser scanning, and X-ray inspection. Each technique offers its unique advantages and capabilities, allowing manufacturers to tailor their inspection processes to meet specific application requirements and regulatory guidelines. By staying abreast of the latest advancements in bottle inspection technology, pharmaceutical companies can ensure that their quality control efforts remain at the forefront of industry best practices.
Setting the Standard: Bottle Inspection Criteria and Standards
Bottle inspection criteria and standards serve as the foundation for quality control in pharmaceutical manufacturing, providing clear guidelines for assessing the integrity and safety of containers. From dimensional specifications to visual appearance criteria, these standards outline the requirements that bottles must meet to be deemed acceptable for use. Automated bottle inspection systems play a crucial role in enforcing these standards by providing objective measurements and assessments of bottle quality, thereby ensuring compliance with regulatory requirements and industry best practices.
Harnessing the Power of Bottle Inspection Software
Bottle inspection software plays a central role in the operation of automated inspection systems, providing a user-friendly interface for configuring inspections, analyzing results, and generating reports. These software platforms leverage advanced algorithms and data visualization techniques to streamline the inspection process, facilitate data-driven decision-making, and optimize overall efficiency and accuracy. By investing in robust bottle inspection software, pharmaceutical manufacturers can maximize the capabilities of their automated inspection systems and ensure the reliability and consistency of their quality control efforts.
Ensuring Accuracy in Bottle Inspection
Accuracy is paramount in bottle inspection, as even the smallest deviation from quality standards can have significant implications for product safety and regulatory compliance. Automated inspection systems leverage advanced sensor technology and precise measurement algorithms to achieve unparalleled levels of accuracy in detecting defects, verifying dimensions, and assessing overall bottle quality. By providing objective, data-driven assessments of bottle integrity, these systems enable pharmaceutical manufacturers to minimize the risk of product recalls, protect consumer health, and maintain the trust and confidence of regulatory authorities and consumers alike.
Conclusion:
In conclusion, automated bottle inspection systems represent a transformative advancement in pharmaceutical quality control, offering unmatched accuracy, efficiency, and reliability in detecting defects and ensuring product integrity. By harnessing the power of machine vision technology and innovative inspection techniques, these systems enable pharmaceutical manufacturers to uphold the highest standards of quality and safety while maximizing productivity and efficiency in their operations. As the industry continues to evolve, automated bottle inspection will play an increasingly vital role in shaping the future of pharmaceutical manufacturing, driving innovation, and excellence in quality assurance.