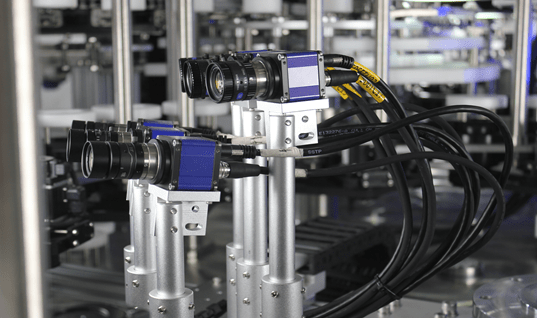
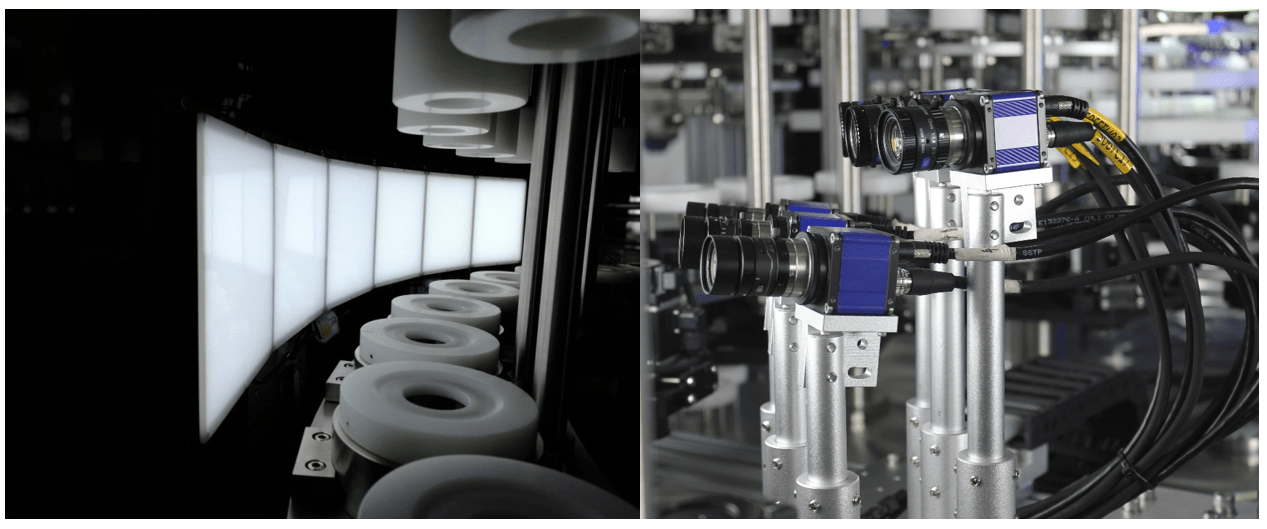
Automatic Visual Inspection Machines
In the pharmaceutical industry, ensuring the safety and quality of parenteral products is paramount. Any deviation from quality standards can have severe consequences for patients’ health. Among the various quality control measures employed, automatic visual inspection machines play a crucial role in detecting defects and contaminants in parenteral products. This article explores the significance of automatic visual inspection machines in enhancing quality assurance within the realm of parenteral product manufacturing.
Introduction
Parenteral products, including injectables, infusions, and vaccines, are administered directly into the body through routes other than the digestive tract. Due to their direct interaction with the bloodstream, these products must meet stringent quality standards to ensure patient safety. Even minor defects or contaminants can lead to adverse effects, making quality control processes indispensable.
The Importance of Visual Inspection
Visual inspection is a fundamental aspect of quality control in pharmaceutical manufacturing. It involves the meticulous examination of products for defects such as cracks, chips, particles, and discoloration. While manual inspection has been the traditional approach, it is prone to human error and subjectivity, making it less reliable, especially for high-volume production.
Advantages of Automatic Visual Inspection Machines
Automatic visual inspection machines offer several advantages over manual inspection:
Precision and Consistency: These machines use advanced imaging technology to detect even the minutest defects with high precision and consistency, eliminating the variability associated with human inspectors.
High-Speed Inspection: Automatic machines can inspect thousands of units per hour, ensuring efficient quality control without compromising production speed.
Comprehensive Inspection: They can examine various aspects of the product, including size, shape, color, and integrity, providing a comprehensive assessment of quality.
Data Recording and Analysis: Automatic inspection machines can record and analyze inspection data in real-time, enabling manufacturers to identify trends, troubleshoot issues, and improve processes proactively.
Reduced Risk of Contamination: By minimizing human intervention, automatic inspection machines help reduce the risk of contamination, ensuring product safety and compliance with regulatory standards.
Application in Parenteral Product Manufacturing
In the context of parenteral product manufacturing, automatic visual inspection machines play a critical role at various stages of production:
Incoming Raw Materials: These machines can inspect incoming vials, ampoules, and other primary packaging materials for defects before they are used in production, preventing the introduction of faulty components into the manufacturing process.
In-Process Inspection: During the manufacturing process, automatic inspection machines can inspect filled containers for defects such as particulate matter, container closure integrity, and fill level accuracy, ensuring that only high-quality products proceed to the next stage.
Final Product Inspection: Before packaging and distribution, parenteral products undergo a final inspection to ensure they meet quality standards. Automatic visual inspection machines play a crucial role in this stage by detecting any defects or contaminants that may have occurred during production.
Regulatory Considerations
Regulatory agencies such as the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have stringent requirements for the inspection of parenteral products. Manufacturers must demonstrate that their inspection processes, including the use of automatic visual inspection machines, are capable of detecting defects and contaminants in accordance with regulatory standards.
Challenges and Limitations
While automatic visual inspection machines offer significant benefits, they are not without challenges and limitations:
Complexity of Inspection: Some defects, such as sub-visible particles or cosmetic imperfections, may be challenging for automatic machines to detect accurately, requiring additional manual inspection or supplementary analytical techniques.
Validation and Qualification: Validating and qualifying automatic inspection machines can be time-consuming and resource-intensive, requiring thorough testing to ensure their performance meets regulatory requirements.
Cost of Implementation: The initial investment and ongoing maintenance costs associated with automatic inspection machines can be substantial, particularly for smaller pharmaceutical companies with limited resources.
Future Trends
Despite these challenges, the adoption of automatic visual inspection machines in parenteral product manufacturing is expected to grow in the coming years. Key trends driving this growth include:
Advancements in Technology: Continued advancements in imaging technology, including machine learning and artificial intelligence, will enhance the capabilities of automatic inspection machines, enabling more accurate and efficient defect detection.
Integration with Industry 4.0: Automatic inspection machines are increasingly being integrated with other smart manufacturing technologies as part of Industry 4.0 initiatives, enabling real-time monitoring, data analytics, and predictive maintenance.
Focus on Patient Safety: With an increasing focus on patient safety and regulatory compliance, pharmaceutical companies are investing in robust quality control systems, including automatic inspection machines, to ensure the integrity of parenteral products.
Conclusion
Automatic visual inspection machines play a vital role in enhancing quality assurance in parenteral product manufacturing. By leveraging advanced imaging technology, these machines offer precision, speed, and reliability in defect detection, thereby safeguarding patient safety and ensuring compliance with regulatory standards. As technology continues to evolve and regulatory requirements become more stringent, the adoption of automatic inspection machines is poised to become increasingly widespread, driving improvements in the quality and safety of parenteral products.
Inspection Machine