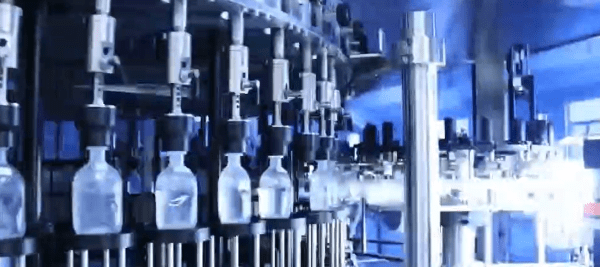
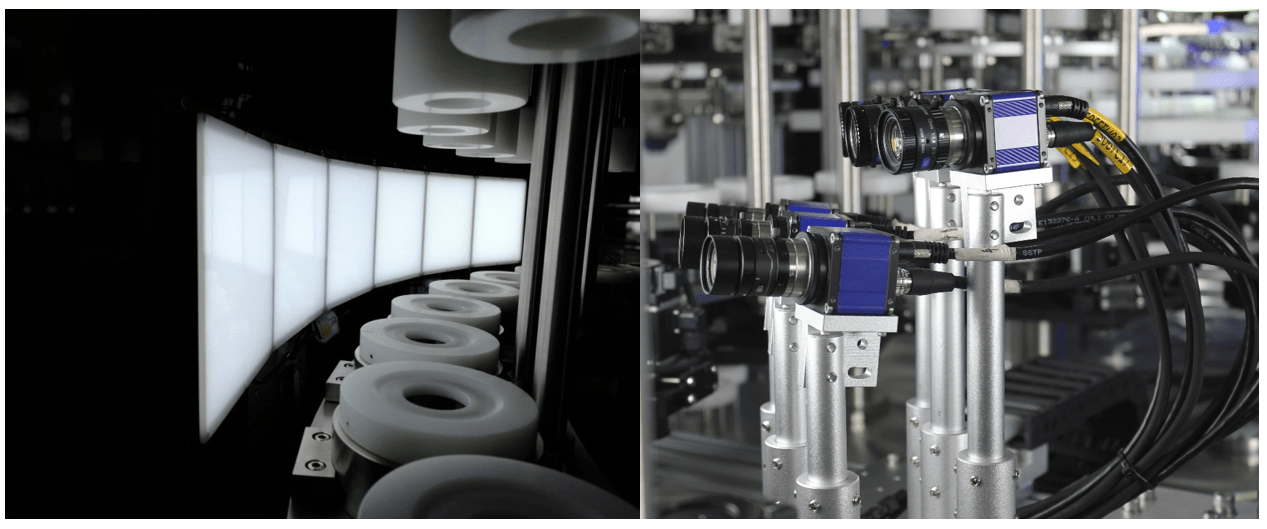
Introduction
Automated Visual Inspection (AVI) systems play a crucial role in the pharmaceutical industry, particularly in the inspection of parenteral solutions. These solutions, intended for injection, must meet the highest standards of quality and safety due to their direct administration into the human body. Contamination in parenteral solutions can lead to severe health risks, including infections, allergic reactions, and even life-threatening conditions. Therefore, the implementation of AVI systems is essential to ensure that each product is free from any form of contamination before reaching the patient.
Components of AVI Systems
Imaging Technology
1. Cameras:
– High-resolution cameras are the backbone of AVI systems. They capture detailed images of the parenteral solutions, allowing for precise detection of contaminants. The quality of these cameras directly impacts the system’s ability to identify even the smallest particles.
2. Lighting:
– Proper lighting is critical for highlighting contaminants within the solution. Various lighting techniques, such as backlighting, front lighting, and side lighting, are employed to enhance visibility. For instance, backlighting can make particles within the solution more visible by creating a contrast against the light source.
3. Lenses:
– Different lenses are used to focus on various aspects of the product. Macro lenses can be used for close-up inspections of the solution and container, while wide-angle lenses might be employed to capture the entire container for integrity checks.
Conveyor Systems
– Parenteral containers such as vials, ampoules, or syringes are moved along a conveyor belt, passing through the inspection area. The conveyor system ensures that each container is correctly positioned and oriented for optimal inspection by the cameras and lighting systems.
Software and Algorithms
1. Image Processing:
– Advanced image processing algorithms are used to analyze the captured images. These algorithms can detect anomalies such as foreign particles, discoloration, and container defects by comparing the images to predefined quality standards.
2. Machine Learning:
– Machine learning models enhance the system’s accuracy over time. By learning from previous inspection results, these models can improve their ability to detect defects and reduce false positives. This continuous learning process allows the system to adapt to new types of defects and variations in the production process.
Rejection Mechanisms
– Defective products are automatically removed from the production line. This rejection mechanism can include air jets, mechanical arms, or diverter systems that segregate the defective containers without interrupting the flow of production.
Types of Contaminations Detected
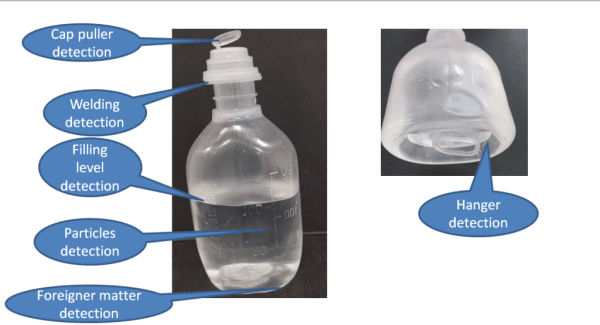
1. Particulate Matter:
– AVI systems can detect foreign particles such as dust, fibers, glass, or metal shavings. These particles can enter the solution during the manufacturing process or from the environment.
2. Liquid Contaminants:
– Discoloration or the presence of unexpected substances in the solution can indicate contamination. AVI systems are designed to identify any changes in the color or clarity of the solution.
3. Container Defects:
– Structural defects in the containers, such as cracks, chips, or improper seals, are also detected. These defects can compromise the sterility and integrity of the parenteral solution.
4. Fill Level Issues:
– AVI systems ensure that each container is filled to the correct level. Underfilled or overfilled containers can indicate problems in the filling process and may not meet regulatory standards.
Inspection Process
Preparation
– Before inspection, containers are cleaned and prepared to remove any surface contaminants. This preparation ensures that the AVI system can accurately assess the solution inside the container without interference from external particles.
Imaging
– Containers pass through the imaging station, where multiple images are captured from different angles. This multi-angle approach ensures comprehensive coverage of the container and its contents, increasing the likelihood of detecting any contaminants or defects.
Analysis
– The captured images are analyzed in real-time using sophisticated software. The analysis involves comparing the images to reference standards and identifying any deviations. This process can include pattern recognition, edge detection, and color analysis techniques.
Decision Making
– Based on the analysis, the system decides whether each container passes or fails the inspection. Containers that meet the quality standards continue along the production line, while those that do not are flagged for rejection.
Rejection
– Rejected containers are automatically removed from the production line. The rejection mechanism ensures that only products meeting the highest quality standards proceed to packaging and distribution.
Advantages of AVI
Consistency and Reliability
– AVI systems provide a consistent level of inspection quality, free from the variability associated with human inspectors. Human inspectors can become fatigued, leading to errors, while AVI systems can operate continuously with the same level of precision.
Speed
– Automated systems can inspect products at a much faster rate than manual inspection, significantly increasing throughput. This speed is essential in high-volume production environments where delays can be costly.
Cost-Effectiveness
– Over time, AVI systems reduce labor costs and minimize the risk of costly recalls due to undetected contaminants. While the initial investment in AVI technology can be high, the long-term savings and benefits outweigh the costs.
Compliance
– AVI systems help maintain regulatory compliance by ensuring products meet stringent quality standards. Regulatory bodies such as the FDA and EMA require rigorous quality control measures for parenteral solutions, and AVI systems help manufacturers meet these requirements.
Challenges and Considerations
Initial Setup Costs
The initial investment for purchasing and setting up AVI systems can be substantial. However, this cost is offset by the long-term benefits of improved quality control and reduced labor costs.
Complexity of Inspection
– Developing and fine-tuning algorithms to detect all possible defects can be complex. This process requires significant expertise in both software development and pharmaceutical manufacturing.
Maintenance
– Regular maintenance is required to ensure the system remains accurate and effective. This maintenance includes calibrating cameras, updating software, and replacing worn-out components.
Regulatory Compliance
-Compliance with regulatory standards is critical in the pharmaceutical industry. AVI systems help companies meet these standards by providing detailed inspection reports and documentation. These reports can be used to demonstrate compliance during regulatory audits.
Technological Advancements
-Continuous advancements in imaging technology and machine learning algorithms are further enhancing the capabilities of AVI systems. For instance, the integration of artificial intelligence (AI) has improved the accuracy and speed of defect detection, making AVI systems more effective and reliable.
Future Trends in AVI for Parenteral Solutions
Artificial Intelligence and Machine Learning
– The future of AVI lies in the integration of AI and machine learning. These technologies can significantly improve the accuracy and efficiency of defect detection by learning from vast amounts of data. AI can also predict potential defects based on historical data, allowing for proactive quality control measures.
Enhanced Imaging Techniques
-Advances in imaging technology, such as hyperspectral imaging and 3D imaging, are set to revolutionize AVI systems. Hyperspectral imaging can detect contaminants based on their spectral signatures, while 3D imaging provides a more comprehensive view of the container and its contents.
Automation and Integration
– Greater automation and integration with other manufacturing processes will further streamline quality control. For example, integrating AVI systems with manufacturing execution systems (MES) can provide real-time feedback and adjustments, ensuring consistent product quality throughout the production process.
Regulatory Evolution
– As regulatory standards evolve, AVI systems will need to adapt to meet new requirements. This adaptation will involve continuous updates to inspection algorithms and compliance documentation to ensure that AVI systems remain effective and compliant.
Conclusion
Automated Visual Inspection is indispensable for ensuring the quality and safety of parenteral solutions. By leveraging advanced imaging technology, sophisticated software, and efficient mechanical systems, AVI systems provide a reliable and efficient means of detecting contamination and defects. The advantages of AVI, including consistency, speed, cost-effectiveness, and compliance, make it a vital component of modern pharmaceutical manufacturing. Despite the challenges of initial setup costs, complexity, and maintenance, the long-term benefits of AVI systems in maintaining product quality and protecting patient safety are substantial. As technology continues to advance, the capabilities of AVI systems will only improve, further enhancing their role in the pharmaceutical industry.
Inspection Machine