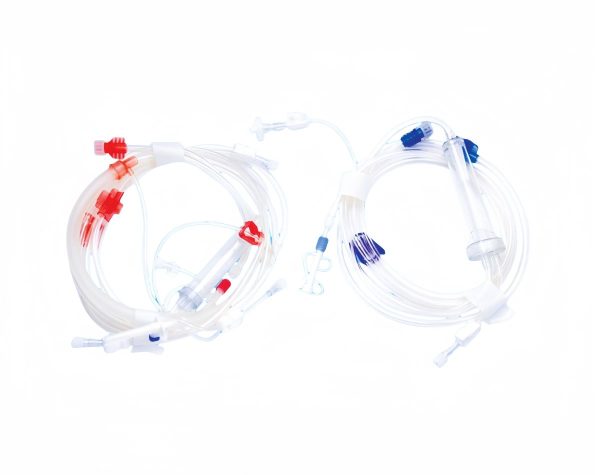
The automated hemodialysis tube assembly machine is a sophisticated piece of equipment designed to ensure the efficient, precise, and consistent production of hemodialysis tubing sets. These machines incorporate advanced automation technologies to meet the high standards required for medical devices. This document provides a detailed overview of the assembly process, inspection procedures, and the technologies involved in ensuring the quality and reliability of the hemodialysis tubes.
Assembly Process
1. Material Handling and Preparation
1.1 Raw Material Feeding:
The process begins with the automated feeding of raw materials into the assembly machine. The key materials include:
- Tubing: Typically made from medical-grade PVC or silicone.
- Connectors: Used to connect the tubing to dialysis machines and other medical equipment.
- Clamps and Seals: These components are crucial for controlling fluid flow and ensuring the integrity of the tubing set.
1.2 Automated Spools:
The tubing material is often supplied on large spools. The automated machine unwinds these spools and feeds the tubing into the assembly line. This automated handling ensures a continuous and efficient supply of tubing, reducing downtime and manual intervention.
2. Cutting and Measurement
2.1 Precision Cutting:
The tubing is cut to specified lengths using precision cutting tools, such as automated blades or laser cutters. The cutting process is controlled by sophisticated software that ensures each piece of tubing is cut to the exact required length.
2.2 Sensors and Measurement Tools:
Advanced sensors and measurement tools continuously monitor the length and quality of the cut tubing. These tools detect any deviations from the specified dimensions and tolerances, ensuring uniformity and high quality in the final product.
3. Assembly and Connection
3.1 Automated Connectors Attachment:
Robotic arms or other automated mechanisms position and secure connectors to the ends of the cut tubing. This step may involve:
- Insertion: The connectors are inserted into the tubing ends with high precision.
- Welding/Bonding: Methods such as ultrasonic welding or adhesive bonding are used to create a secure and sterile connection between the tubing and connectors.
3.2 Integration of Clamps and Seals:
Clamps and seals are precisely positioned and secured onto the tubing assembly. These components are crucial for the functionality and reliability of the hemodialysis tubing sets, ensuring they can control fluid flow and prevent leaks.
Inspection and Quality Control
Quality control is a critical aspect of the hemodialysis tube assembly process. Automated inspection systems are integrated into the assembly line to ensure each product meets stringent medical standards. Key inspection procedures include leak testing, block detection, and comprehensive inspections by advanced machines.
1. Leak Testing
1.1 Leak Test Devices:
Leak test devices are used to verify the integrity of the tubing assemblies. These devices detect any leaks that could compromise the functionality and safety of the hemodialysis tubing. The leak test typically involves the following steps:
- Sealing the Ends: The ends of the tubing assembly are sealed, and the tubing is pressurized with air or another test gas.
- Pressure Monitoring: The pressure inside the tubing is monitored over a set period. Any drop in pressure indicates a potential leak.
- Automated Detection: Automated systems detect leaks with high sensitivity, ensuring even small leaks are identified and addressed.
1.2 Inline Leak Testing:
Inline leak testing is integrated into the assembly process, allowing for continuous monitoring and immediate detection of leaks. This approach ensures that defective tubing is identified and removed from the production line promptly.
2. Block Detection
2.1 Sensors for Block Detection:
Block detection sensors are used to ensure there are no blockages within the tubing that could hinder fluid flow during dialysis. These sensors work by:
- Flow Measurement: Measuring the flow of air or a test fluid through the tubing.
- Pressure Drop Analysis: Analyzing any pressure drops that may indicate a blockage.
- Automated Alerts: Providing automated alerts if a blockage is detected, allowing for immediate corrective action.
2.2 Inline Block Detection:
Inline block detection is crucial for maintaining the efficiency of the production process. By continuously monitoring for blockages, the system ensures that only fully functional tubing assemblies proceed to the next stages of production.
3. Inspection Machines
3.1 High-Resolution Cameras:
Inspection machines equipped with high-resolution cameras perform visual inspections of the tubing assemblies. These cameras are capable of detecting:
- Surface Defects: Such as scratches, cuts, or deformations.
- Misalignments: Ensuring connectors, clamps, and seals are correctly positioned.
- Contaminants: Identifying any foreign particles or contaminants on the tubing surface.
3.2 Advanced Imaging Technologies:
Technologies such as infrared imaging or X-ray inspection may be used to detect internal defects that are not visible to the naked eye. These advanced imaging methods ensure comprehensive inspection of the tubing assemblies.
3.3 Automated Inspection Systems:
Automated inspection systems are integrated into the assembly line to provide real-time monitoring and quality control. These systems use sophisticated algorithms to analyze the data collected by sensors and cameras, ensuring immediate identification and correction of defects.
Final Quality Assurance
1. Sterilization
1.1 Sterilization Methods:
The assembled and inspected tubing sets undergo sterilization to ensure they are safe for medical use. Common sterilization methods include:
- Ethylene Oxide (EtO) Sterilization: Suitable for materials sensitive to heat and moisture.
- Gamma Irradiation: Using gamma rays to sterilize the tubing without leaving any residues.
1.2 Sterilization Verification:
After sterilization, samples from each batch are tested to verify the effectiveness of the sterilization process. This step ensures that the tubing sets are free from harmful microorganisms.
2. Packaging and Labeling
2.1 Automated Packaging:
The sterilized tubing sets are packaged in sterile conditions to maintain their sterility until use. Automated packaging systems handle this process, sealing the tubing in sterile bags or pouches.
2.2 Accurate Labeling:
Automated labeling systems print and apply labels to each package. Labels include essential information such as product details, lot numbers, expiration dates, and regulatory compliance marks. Accurate labeling is critical for traceability and compliance with medical standards.
3. Final Inspection and Distribution
3.1 Final Inspection:
Before distribution, a final inspection is conducted to ensure all packaged products meet quality standards. This inspection includes:
- Visual Checks: Ensuring package integrity and proper labeling.
- Functional Verification: Confirming that the tubing sets are ready for use.
3.2 Distribution:
The final products are packed into cartons and prepared for distribution. Distribution channels include direct shipments to hospitals, dialysis centers, or medical supply distributors.
Key Points and Benefits of Automation
Increased Efficiency:
- Automation significantly reduces the time required to produce hemodialysis tubing sets, leading to higher production rates and improved response to market demand.
Enhanced Precision:
- Automated systems ensure consistent product quality with minimal human error. Precision in cutting, assembling, and inspecting the tubing sets is maintained throughout the process.
Cost Reduction:
- Although the initial investment in automation technology is substantial, the reduction in labor costs and increased production efficiency result in long-term savings. Automation also reduces material waste, further lowering production costs.
Scalability:
- Automated machines can be scaled to meet increasing demand, allowing manufacturers to adjust production volumes as needed. This scalability is essential for responding to market needs and expanding production capacity.
Compliance and Traceability:
- Automated systems are designed to comply with stringent regulatory standards, including those set by the FDA and other international bodies. They include traceability features that track production batches, ensuring accountability and quality control.
Improved Safety:
- Automation reduces the need for manual handling, minimizing the risk of contamination and ensuring a sterile production environment. This is particularly important for medical devices that come into direct contact with patients.
Customization:
- Automated machines can be customized to handle different types of tubing sets or to incorporate specific features required by medical practitioners. This flexibility allows manufacturers to meet diverse customer needs.
Industry Applications
Automated hemodialysis tube assembly machines are utilized by medical device manufacturers who produce consumables for dialysis treatment. These consumables are vital for dialysis centers, hospitals, and home dialysis systems, ensuring a reliable supply of high-quality, sterile tubing sets for patients with kidney failure. By incorporating advanced automation technology, manufacturers can meet the stringent quality standards required for medical devices while optimizing their production processes to handle the growing demand for hemodialysis treatments worldwide.
Conclusion
The automation of hemodialysis tube assembly represents a significant advancement in medical manufacturing. The integration of sophisticated automation technologies ensures that tubing sets are produced with high precision, efficiency, and compliance with regulatory standards. This not only improves the quality and reliability of the products but also enhances the overall efficiency and scalability of the production process. As the demand for hemodialysis treatments continues to grow, the role of automated assembly machines will become increasingly vital in meeting global healthcare needs.
Dialysis Blood Tube Assembly Line