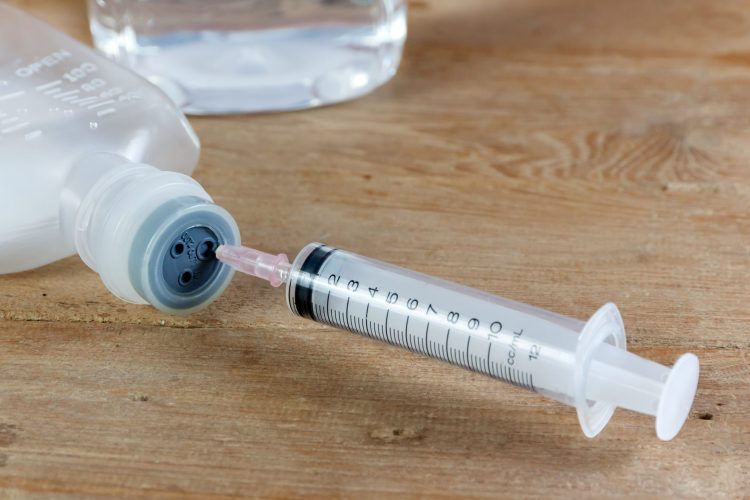
Rubber for IV solution packaging plays a crucial role in revolutionizing healthcare safety and durability. Medical grade rubber materials are used in the production of various components of IV solution packaging, such as rubber stoppers, seals, safety caps, and elastomeric closures. These materials are essential for ensuring the containment, preservation, and protection of IV solutions.
Rubber used in IV packaging is designed to be leak proof, safe for IV storage, and durable to withstand the rigors of healthcare settings. It is crucial that rubber seals and closures used in IV packaging meet stringent medical packaging standards to ensure the safety and efficacy of the IV solutions they contain.
Innovations in rubber packaging have led to the development of latex free packaging for individuals with latex allergies. Biocompatible rubber materials are also used to ensure compatibility with pharmaceutical products and provide a sterile environment for IV solutions.
The production of rubber for IV solution packaging involves stringent sterilization processes to maintain the integrity of the packaging and prevent contamination. Rubber packaging for IV solution bottles undergoes thorough testing to ensure that it meets quality and safety standards for healthcare packaging.
Overall, rubber for IV solution packaging plays a vital role in ensuring the safety, durability, and efficacy of healthcare packaging. It continues to evolve with advancements in medical rubber products, making it a key component in the pharmaceutical industry.
Understanding the Necessity of IV Solution Packaging
IV solution packaging is essential in the medical and pharmaceutical industries to ensure the safety and efficacy of intravenous solutions. Medical grade rubber materials play a crucial role in the production of sterile packaging for IV solutions. Rubber stoppers, seals, and safety caps are commonly used components in IV bag seals to prevent contamination and leakage.
Latex free packaging is becoming more prevalent to cater to individuals with latex allergies. It is important to adhere to medical packaging standards to guarantee the quality and safety of IV solution containment. Rubber durability and compatibility are key factors in selecting IV packaging materials to preserve the integrity of the solution.
Elastomeric closures made from biocompatible rubber are widely used in healthcare packaging to maintain the sterility of IV solutions. Innovations in rubber packaging have led to the development of leak proof seals for safe IV storage. Rubber sterility and compatibility are crucial for ensuring the preservation of IV solutions.
Durable medical packaging made from rubber ensures the protection of IV solution bottles during storage and transportation. The production of medical rubber products follows strict guidelines to meet pharmaceutical standards. By utilizing high quality rubber materials, healthcare providers can guarantee the safety and efficacy of IV solutions.
Exploring Rubber Materials in Pharmaceutical Packaging
Rubber materials play a crucial role in pharmaceutical packaging, especially in IV solution packaging. Medical grade rubber is commonly used in sterile packaging to ensure the safety and preservation of IV solutions. Rubber stoppers and seals are essential components of IV bags, providing leak proof seals and safe storage for IV solutions.
Latex free packaging options are available to address potential allergies, while rubber safety caps help maintain the integrity of IV solutions. The durability of rubber ensures that medical packaging meets industry standards for safe containment and preservation of IV solutions.
Elastomeric closures made from rubber offer compatibility with various IV packaging materials, ensuring a secure fit. Rubber sterilization techniques are employed to maintain the cleanliness and biocompatibility of pharmaceutical rubber products.
Innovations in rubber packaging have led to the development of technologically advanced rubber products for healthcare packaging. Biocompatible rubber materials provide protection for IV solutions, meeting the stringent requirements of pharmaceutical packaging.
Overall, rubber materials play a vital role in ensuring the safety, durability, and effectiveness of IV solution packaging in the healthcare industry.
For more information on rubber materials in pharmaceutical packaging, please visit the Wikipedia page on pharmaceutical packaging.
The Role of Medical Grade Rubber in Sterile Packaging
Medical grade rubber plays a crucial role in sterile packaging for IV solution containment. Rubber materials are commonly used in pharmaceutical packaging, particularly in IV bag components such as rubber stoppers and seals. These components ensure leak proof packaging and safe storage of IV solutions. Latex free packaging is also essential to prevent allergic reactions in patients, making rubber safety caps and seals a vital part of medical packaging standards.
The durability of medical grade rubber is paramount in ensuring the preservation of IV solutions. Elastomeric closures made from rubber provide a secure seal, protecting the contents from contamination. Rubber compatibility with IV packaging materials is crucial for maintaining the integrity of the solution.
Innovations in rubber production have led to the development of biocompatible rubber products that are suitable for healthcare and pharmaceutical use. Medical rubber products, such as rubber packaging for IV solution bottles, adhere to stringent quality standards to ensure the safety and efficacy of medical treatments.
Sterilization of rubber components is a critical process in manufacturing sterile packaging for IV solutions. Ensuring that rubber seals and closures are sterile is essential for maintaining the sterility of the contents.
Overall, the role of medical grade rubber in sterile packaging is indispensable for the protection and preservation of IV solutions, contributing to the safe delivery of healthcare treatments to patients.
Components of IV cap: The Importance of Rubber Stoppers
Rubber stoppers play a crucial role in IV solution packaging, ensuring the safety and integrity of the contents inside. These stoppers are typically made from medical grade rubber, which is sterile and latex free to meet pharmaceutical packaging standards. When it comes to IV bag components, rubber stoppers act as seals to prevent any leakage and contamination of the IV solution.
Rubber stoppers are essential for IV solution containment, providing a secure and airtight seal for IV bags and bottles. They are designed to be durable and long lasting, ensuring the preservation of the IV solution throughout its shelf life. The elastomeric closures made from rubber offer excellent compatibility with various IV packaging materials, making them an ideal choice for medical packaging.
In addition, rubber stoppers are designed to withstand the sterilization process, ensuring that the IV solution remains safe for patient use. These rubber safety caps are leak proof and provide a secure seal for safe IV storage. The production of rubber stoppers involves innovative techniques to enhance the durability and reliability of medical rubber products for healthcare packaging.
Furthermore, rubber stoppers are biocompatible and pharmaceutical grade, making them ideal for IV solution protection. They are designed to meet the highest standards of quality and safety in medical packaging. With their excellent compatibility with IV solution bottles, rubber stoppers play a crucial role in ensuring the safe administration of medications to patients.
Overall, rubber stoppers are an essential component of IV caps, providing a secure and reliable seal for IV solutions. Their importance in pharmaceutical packaging cannot be understated, as they help maintain the integrity and safety of IV medications. Choose rubber stoppers for leak proof and durable medical packaging solutions that prioritize patient safety and product quality.
Latex Free Packaging: Ensuring Safe IV Solution Containment
Rubber materials play a crucial role in ensuring the safe containment of IV solutions through latex free packaging. Pharmaceutical packaging relies heavily on medical grade rubber for components such as rubber stoppers, seals, safety caps, and IV bag seals. These rubber components are essential for maintaining sterile packaging and preventing leaks, ultimately preserving the integrity of the IV solution.
When it comes to IV solution containment, latex free packaging is vital for individuals with latex allergies or sensitivities. Rubber seals and closures made from biocompatible materials are essential for meeting medical packaging standards and ensuring safe IV storage. The durability of rubber packaging is also important for protecting IV solutions during transportation and storage.
Elastomeric closures made from rubber are designed to be compatible with IV packaging materials, providing a leak proof seal to prevent contamination. Innovations in rubber production have led to the development of safer and more advanced rubber packaging solutions for medical products. Rubber sterilization processes further enhance the safety and reliability of rubber packaging for IV solutions.
IV solution bottles are commonly equipped with rubber packaging to provide a secure and reliable containment system. The use of medical rubber products in healthcare packaging helps to protect IV solutions from external factors and maintain their efficacy. Pharmaceutical rubber is specifically designed to meet the stringent requirements of IV solution protection and preservation.
Overall, rubber plays a crucial role in the development of durable medical packaging for IV solutions, ensuring that they remain safe and effective throughout their shelf life. By utilizing latex free and biocompatible rubber materials, healthcare providers can trust in the reliability and safety of their IV solution containment systems.
The Crucial Role of Rubber Seals in Medical Packaging Standards
Rubber seals play a crucial role in maintaining medical packaging standards, especially in IV solution packaging. These seals are typically made from medical grade rubber to ensure the safety and sterility of pharmaceutical packaging. They are commonly used in IV bag components such as rubber stoppers and seals to contain the IV solution and prevent leakage.
Rubber seals also contribute to latex free packaging, making them suitable for individuals with latex allergies. The durability of rubber seals ensures leak proof packaging for safe IV storage and preservation of IV solutions. These elastomeric closures are compatible with various IV packaging materials and can withstand sterilization processes to maintain their integrity.
Innovation in rubber packaging has led to the development of rubber safety caps and biocompatible rubber materials for healthcare packaging. Pharmaceutical rubber products such as rubber seals for IV solution bottles adhere to strict medical packaging standards to ensure the protection of IV solutions.
Overall, rubber seals play a vital role in maintaining durable and safe medical packaging for the containment and preservation of IV solutions.