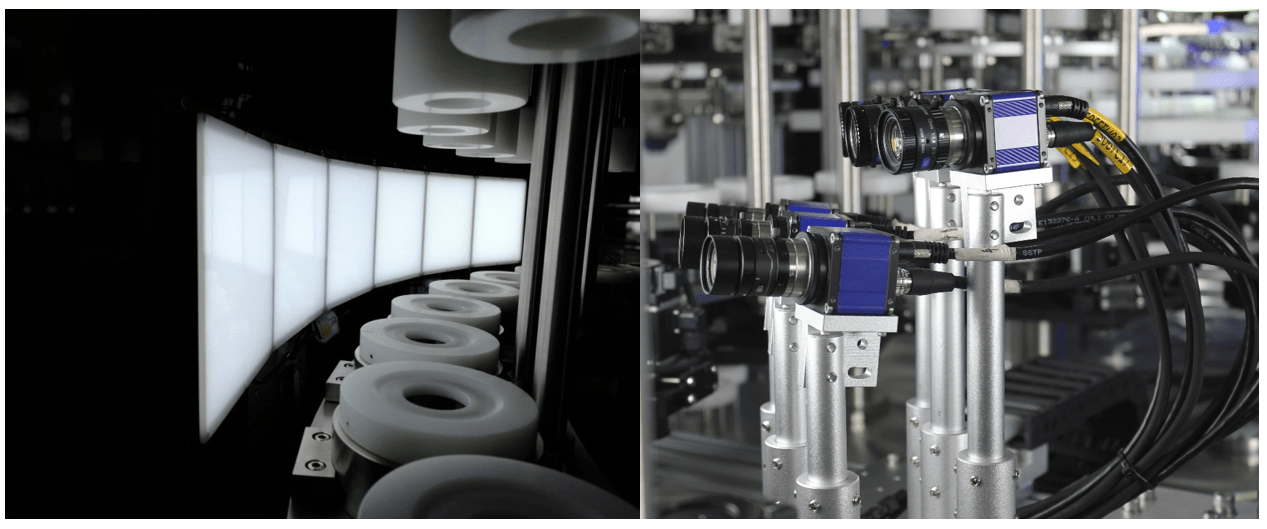
Intravenous (IV) fluid manufacturing demands the utmost precision and quality assurance to ensure the safety and efficacy of these critical medical products. Visual particle detection machines have emerged as indispensable tools in this field, offering advanced capabilities to identify and eliminate contaminants. In this blog post, we delve into the world of visual particle detection machines within IV fluid manufacturing, exploring where these particles originate and how these inspection machines detect them.
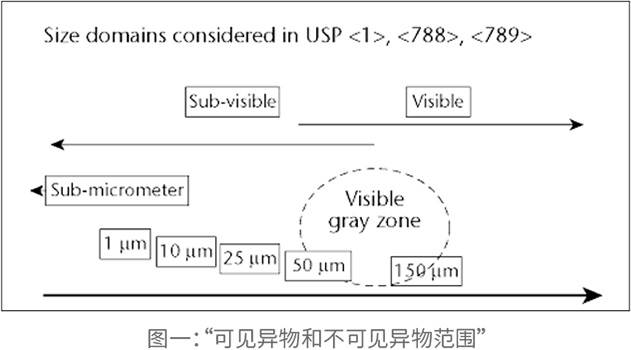
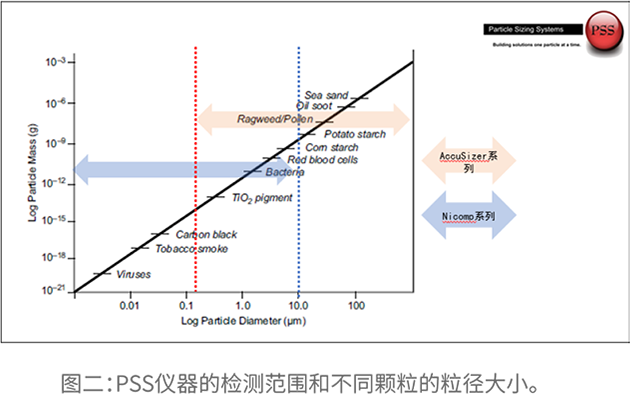
Understanding Particle Contamination in IV Fluid Manufacturing
The production of IV fluids requires a sterile environment and stringent quality control measures to prevent contamination. Despite these precautions, particles can still find their way into the manufacturing process from various sources:
Raw Materials: Particles may originate from the raw materials used in IV fluid production, including water, salts, and additives. Even with thorough filtration and purification processes, trace contaminants can still be present.
Manufacturing Equipment: Equipment used in the manufacturing process, such as mixing tanks, filtration systems, and filling machines, can introduce particles if not properly maintained or cleaned. Wear and tear, as well as the presence of lubricants or residues, can contribute to particle formation.
Packaging Materials: Contaminants can also arise from packaging materials such as vials, stoppers, and seals. Dust, fibers, or residues from the packaging process may inadvertently enter the IV fluid during filling and sealing operations.
Environmental Factors: Environmental conditions within the manufacturing facility, including air quality, temperature, and humidity, can influence the presence of particles. Airborne contaminants or particles from adjacent processes may infiltrate the production area, posing a risk to product integrity.
The Role of Visual Particle Detection Machines
Visual particle detection machines play a crucial role in ensuring the quality and safety of IV fluids by providing comprehensive inspection capabilities. These machines utilize advanced imaging technologies to detect and analyze particles with exceptional accuracy. The detection process typically involves the following steps:
Image Acquisition: High-resolution cameras capture images of the IV fluid as it passes through the inspection system. Multiple angles and lighting conditions may be used to enhance particle visibility.
Image Processing: The captured images undergo digital processing to enhance contrast, remove background noise, and optimize clarity. This preprocessing step prepares the images for accurate particle detection.
Particle Detection Algorithms: Sophisticated algorithms analyze the processed images to identify potential contaminants. These algorithms are trained to recognize particles based on predefined criteria such as size, shape, color, and texture. Machine learning techniques may be employed to improve detection accuracy and adaptability to different types of particles.
Classification and Segmentation: Detected particles are classified and segmented from the background or other features present in the images. This step helps differentiate between contaminants and benign imperfections or air bubbles within the IV fluid.
Reporting and Rejection: Identified particles are logged and categorized based on their characteristics. Depending on predefined thresholds and regulatory requirements, contaminated batches may be automatically rejected from the production line, ensuring that only pristine IV fluids reach the market.
Advantages of Visual Particle Detection Machines in IV Fluid Manufacturing
Visual particle detection machines offer several advantages over traditional inspection methods:
Enhanced Sensitivity: These machines can detect particles as small as a few microns in size, ensuring thorough inspection and compliance with regulatory standards.
Real-time Monitoring: Continuous inspection allows for real-time monitoring of the production process, enabling prompt corrective actions to prevent product defects.
Reduced Waste: By identifying and rejecting contaminated batches early in the manufacturing process, visual particle detection machines help minimize product waste and ensure efficient resource utilization.
Traceability and Documentation: Comprehensive reporting capabilities provide traceability of inspection results, facilitating compliance with regulatory requirements and quality management standards.
Minimized Risk to Patients: By removing contaminants from IV fluids, visual particle detection machines help mitigate the risk of adverse reactions and infections in patients receiving intravenous therapy.
Future Perspectives and Innovations
As the demand for IV fluids continues to grow, visual particle detection machines are poised to undergo further advancements and innovations. Integration with artificial intelligence and machine learning technologies will enable these machines to adapt to evolving particle detection challenges and optimize inspection performance. Additionally, advancements in imaging techniques, such as hyperspectral imaging and 3D imaging, may offer new opportunities for enhancing detection capabilities and improving efficiency in IV fluid manufacturing.
Conclusion
Visual particle detection machines represent a critical component of quality assurance in IV fluid manufacturing, providing advanced capabilities to detect and eliminate contaminants. By leveraging sophisticated imaging technologies and algorithms, these machines ensure the safety and efficacy of IV fluids, thereby safeguarding the well-being of patients. As technology continues to evolve, visual particle detection machines will remain at the forefront of innovation, driving continuous improvement in quality control practices within the pharmaceutical industry.
Visual Inspection Machine