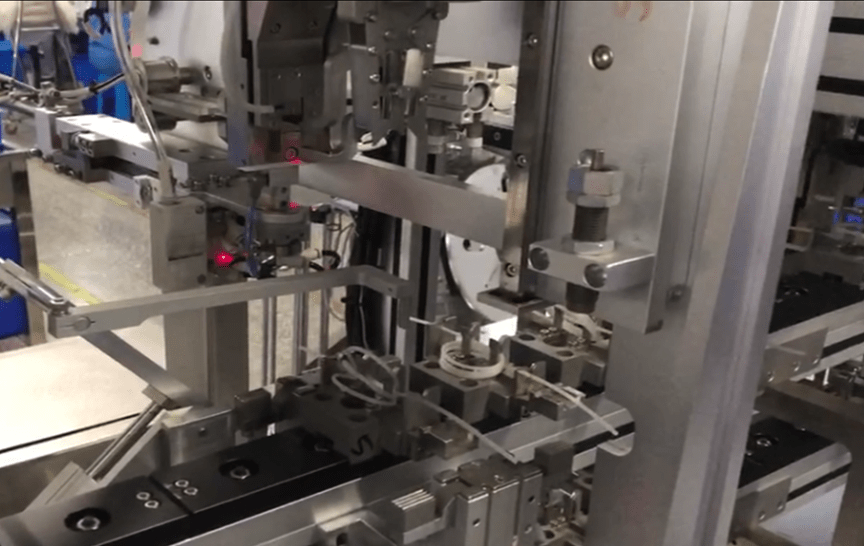
Introduction:
In the realm of medical consumable manufacturing, the quality and precision of catheter production equipment are paramount. Whether you’re a seasoned manufacturer or venturing into the field, understanding the nuances of catheter manufacturing equipment is crucial for achieving optimal results. In this article, we’ll explore key considerations and insights to help you navigate the world of catheter manufacturing equipment effectively.
1. Versatility and Adaptability:
– Catheter manufacturing equipment should offer versatility to accommodate a wide range of catheter designs, sizes, and materials. Look for equipment that can be easily adapted or customized to meet specific manufacturing requirements.
– Flexibility in production capabilities allows for seamless transitions between different catheter types and production volumes, enabling manufacturers to respond quickly to market demands and customer needs.
2. Precision and Consistency:
– Consistent quality is non-negotiable in catheter manufacturing. Seek equipment that delivers precise control over critical parameters such as extrusion rates, molding pressures, and assembly tolerances.
– Advanced automation features and real-time monitoring capabilities help minimize variability and ensure uniformity in catheter dimensions, wall thickness, and performance characteristics.
3. Integration and Efficiency:
– Integration of manufacturing processes is key to optimizing efficiency and streamlining production workflows. Look for equipment that facilitates seamless integration between extrusion, molding, assembly, and quality control processes.
– Modular equipment configurations allow for scalability and expansion as production needs evolve, enabling manufacturers to maximize operational efficiency and minimize downtime.
4. Compliance and Validation:
– Compliance with regulatory standards and validation requirements is paramount in the medical device industry. Choose equipment from reputable manufacturers with a track record of regulatory compliance and adherence to industry standards.
– Equipment should be designed and validated to meet applicable regulatory requirements, including those outlined by regulatory bodies such as the FDA (Food and Drug Administration) and ISO (International Organization for Standardization).
5. Support and Service:
– Opt for equipment suppliers that offer comprehensive support and service packages to ensure smooth implementation, operation, and maintenance of manufacturing equipment.
– Training programs, technical assistance, and ongoing maintenance services are essential for maximizing equipment uptime and optimizing manufacturing performance.
Conclusion:
Investing in the right catheter manufacturing equipment is a critical step towards achieving excellence in catheter production. By prioritizing versatility, precision, integration, compliance, and support, manufacturers can equip themselves with the tools and resources needed to manufacture high-quality catheters efficiently and effectively. As a leading supplier of machines for medical consumable manufacturing, we’re committed to providing innovative solutions and unparalleled support to help manufacturers succeed in their quest to improve patient outcomes and advance medical technology.
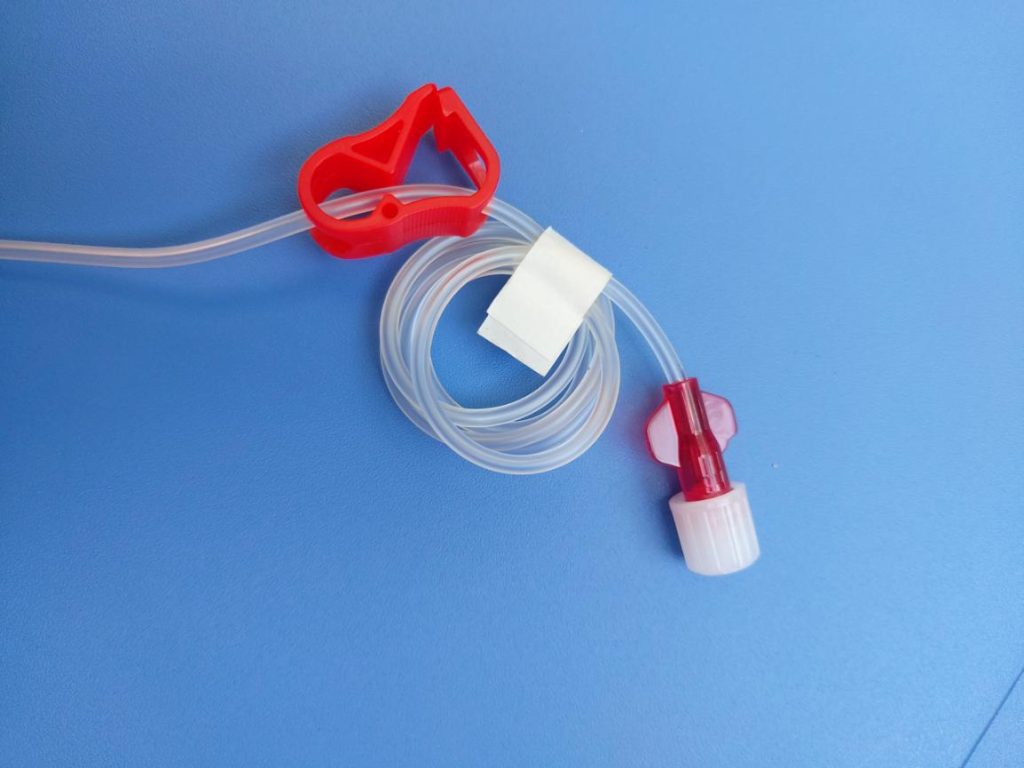
Tube/catheter assembly line